In this week's Antibody Application series, we'll discuss one of the most commonly used techniques in biomedical research: Immunohistochemistry (IHC).
In this article, Moushumi Nathan, a PhD candidate at the University of Toronto, explores the various methods and fixatives used in sample preparation as well as the antibody selection process for an IHC experiment.
What Is Immunohistochemistry (IHC)?
Immunohistochemistry is a technique that uses antibodies (immuno-) in tissues (histo-) to visualize a protein of interest.
There are two main steps in immunohistochemistry:
- Sample preparation: fixing the sample to preserve the architecture of the collected tissue and prepare it for antibody labelling.
- Sample labeling: using antibodies to label the protein(s) of interest.
Sample Preparation
Proper sample preparation is critical to ensure the integrity of the tissue and protein(s) of interest, and to enable antibody detection in the next step.
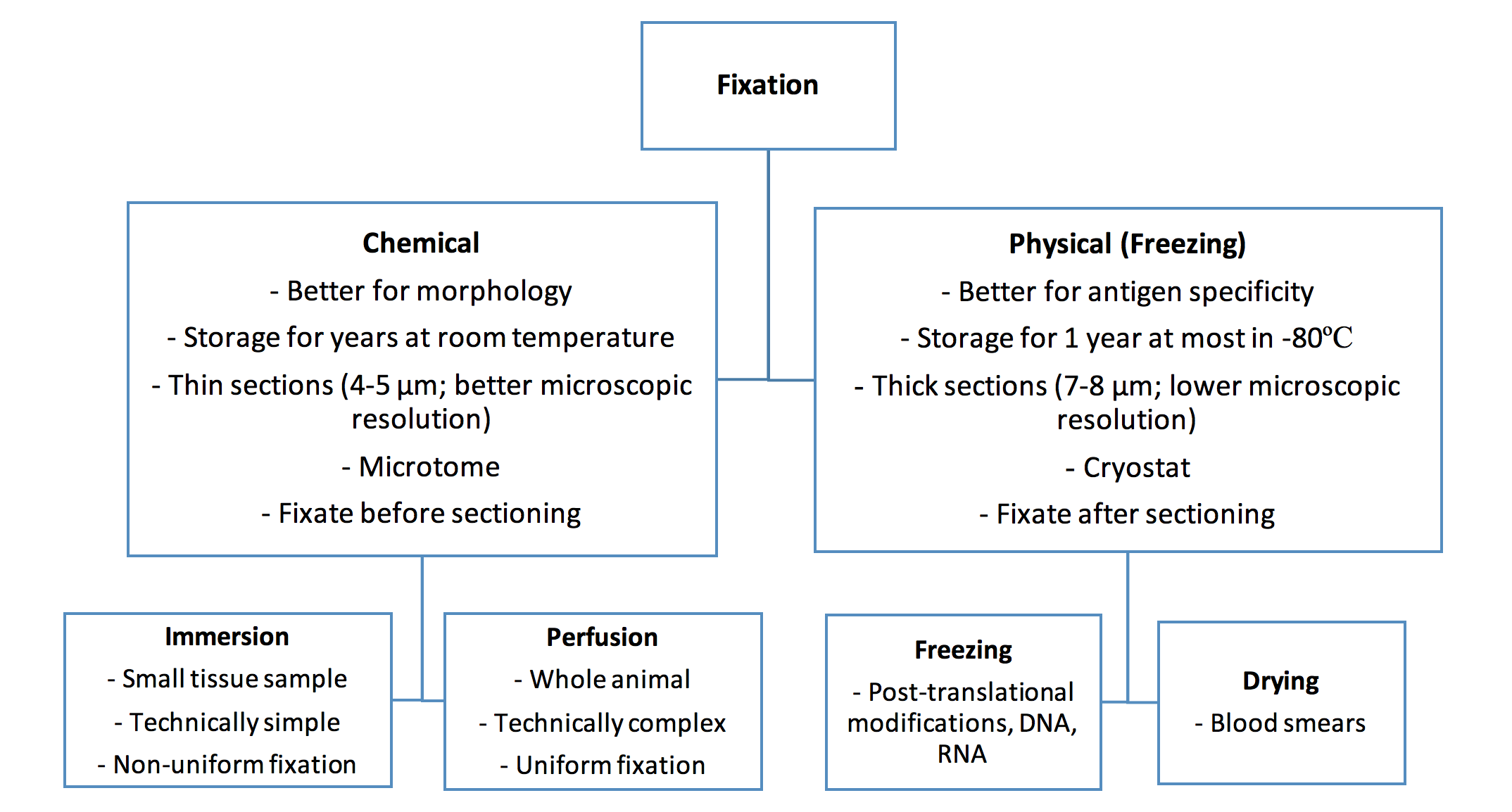
Sample preparation includes fixation, which is the process by which tissues are preserved. The fixation method depends on the type of protein you want to visualize.
I will discuss the different methods of fixation, the different types of fixatives that can be used, and the logic behind the selection of method and type.
Fixation Method Selection
Chemical Fixation
Chemical fixation refers to the use of chemicals to fix tissue. These chemicals can act by either crosslinking proteins or precipitating them, which may mask the antigen, alter antibody accessibility, or change the cellular localization of certain proteins.
Chemical fixation can be done through immersion or perfusion.
Immersion
Immersion consists of immersing the tissue in a large volume of fixative solution (50-100x greater than sample volume). This is ideal for small pieces of tissue (<10mm) to ensure maximal penetration of the fixative across the tissue. Usually, tissues are immersed for 18-24h to prevent underfixation, which may result in tissue degradation and artifacts in antibody labelling, and overfixation, which may mask the antigen and prevent antibody binding.
Immersion can be done in addition to perfusion to ensure full fixation of the tissue of interest.
Perfusion
Perfusion consists of infusing the fixative through the vascular system of the animal, for example that of rodents. This results in rapid and uniform fixative distribution. However, the process of perfusion is technically complex and time consuming compared to immersion. After perfusion, the tissue of interest can be extracted and further fixed through immersion or used in the next step of the protocol.
Physical Fixation
Tissues in which the protein of interest is likely to be affected by the chemicals used in chemical fixation can undergo physical fixation instead, which includes freezing and drying.
Freezing
Freezing consists of embedding tissues in cryogenic materials and then snap-freezing them in liquid nitrogen. Freezing is ideal for monitoring post-translationally modified proteins, DNA, and RNA. However, frozen sections have a limited storage capacity and special storage needs (maximum one year in -80ºC) and tend to be sectioned in thicker sections (cryostat: 7-8 µm versus microtome: 4-5 µm), resulting in poorer microscopic resolution. Furthermore, the formation of ice crystals may influence tissue architecture.
Drying
Drying can be used for certain tissue or fluid types, such as blood smears.
Types of Fixatives
Formalin
Formalin crosslinks amino acids through the formation of methylene bridges. These bridges may affect the epitope of your antigen, thereby influencing your ability to detect it with an antibody. Therefore, antigen retrieval steps may be required to improve antigen detection before sample labeling. Antigen retrieval can be accomplished with either heat-induced epitope retrieval (HIER) or proteolytic-induced epitope retrieval (PIER). Both methods unfold or degrade the crosslinks formed during fixation, thereby reinstating antigenicity.
The native conformations of target proteins, such as tertiary and quaternary structures, may be altered by crosslinking. In addition, DNA sequence alterations and degraded RNA are more frequently observed in formalin fixed tissues. For these reasons, formalin can present issues when detecting post-translationally modified proteins, DNA, or RNA. Instead, formalin is best used when immunostaining for proteins, peptides, and enzymes of a low molecular weight, and for morphological assessments. Formalin is frequently used in immersive fixations.
Paraformaldehyde
Paraformaldehyde also acts by crosslinking proteins, resulting in a fixed insoluble meshwork. Similar to formalin, it may affect the epitope of your antigen, and may require antigen retrieval steps. Paraformaldehyde is best used for immunostaining and morphological assessments of proteins, peptides, enzymes, and small molecules such as amino acids.
Paraformaldehyde is frequently used in both perfusions and immersions.
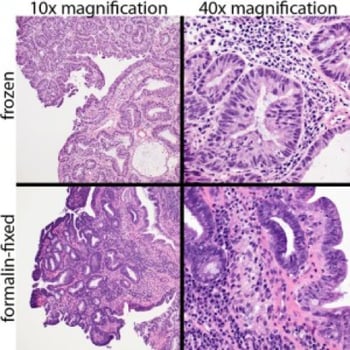
Frozen (top) and formalin-fixed (bottom) images of a Haematoxylin Eosin stained canine rectal papillary adenoma. Cellular resolution is higher in the formalin-fixed section.
Methanol
Methanol acts by precipitating proteins, similar to ethanol and acetone, and can similarly disrupt the epitope structure. Because formalin can interfere with the native conformation of proteins and nucleic acids, methanol (or other alcohols) are preferably used when examining post-translationally modified proteins, RNA, or DNA. Therefore, methanol is frequently used as a fixative after freezing to best assess these molecular features in a tissue. It also works well for large proteins, such as immunoglobulins.
Methanol can also be used to permeabilize the tissue prior to antibody staining.
Paraffin Embedding
To further improve tissue preservation, tissues that have been chemically fixated can then be embedded in paraffin. This is commonly done in conjunction with formaldehyde fixation (FFPE), and allows the sample to be stored at room temperature for years. However, tissues must be de-paraffinated prior to staining, since the hydrophobic nature of paraffin would interfere with antibody staining in aqueous solutions.
| Fixative | Proteins | Mechanism | Perfusion | Immersion | Post-freezing Fixation |
| Formalin | Most proteins, peptides, and enzymes | Covalent cross-link | Yes | ||
| Paraformaldehyde | Most proteins, peptides, enzymes, and small peptides | Covalent cross-link | Yes | Yes | |
| Methanol | Post-translationally modified proteins, large proteins (Igs), DNA, and RNA | Precipitation | Yes | ||
| Paraffin Embedding | Most proteins, peptides, and enzymes | *Yes |
*Frequently combined with formalin fixation (FFPE)
Blocking Non-Specific Binding
Before labelling the protein of interest with an antibody, it's important to perform a blocking step to reduce non-specific antibody binding (false positives). This is achieved by incubating the tissue with solutions containing proteins or other molecules that will bind to readily reactive nonspecific sites, thereby blocking the antibody’s ability to bind to these nonspecific sites. This process is referred to as blocking, and is performed prior to antibody treatment. Common blocking solutions include normal serum and protein solutions, such as bovine serum albumin. Blocking efficacy evaluations can be done by performing negative control experiments (tissues known to not express the antigen of interest).
In addition to blocking non-specific binding of the antibody, it may be necessary to block endogenous expression of certain enzymes, such as peroxidase and alkaline phosphatase, and endogenous molecules, such as biotin. The need for this blocking step will be determined by what the antibody is conjugated to. For example, if a secondary antibody is conjugated to horseradish peroxidase, then we would want this antibody to detect peroxidase specific to the primary antibody, and not all endogenous peroxidase that may be present in the tissue. The need for this blocking step is also determined by the tissue type, since the endogenous expression of these enzymes and molecules are variable among tissue types. For example, blocking endogenous peroxidase activity is important in kidney tissue where there is high peroxidase activity, but not in adipose tissue.
Below is a table listing different blocking types, the corresponding frequently used solutions, use depending on the identity of the antibody to be applied, and tissue types in which these blocking solutions can be performed or may be necessary.
| Blocking Type | Blocking Solution | 2' Antibody | Tissue |
| Non-Specific Binding | Normal serum (ie Goat serum) | 2' antibody host corresponding to serum type (ie Goat anti-mouse) | Any |
| Non-Specific Binding | Protein solutions. ie. Bovine serum albumin, non-fat dry milk | Any | |
| Endogenous Peroxidase | 3-10% hydrogen peroxide | HRP-conjugated antibody | Kidney, liver, and other vascular areas |
| Endogenous Alkaline Phosphatase | 1mM Levamisole | AP-conjugated antibody | Intestine, kidney, lymph |
| Endogenous Biotin | Avidin and biotin | Biotylinated antbody | Liver, kidney, heart, brain, lung |
Antibody Selection
Primary antibodies are used to detect the antigen of interest. They may be conjugated to a fluorophore or a chromogenic enzyme that allows for visualization of the antibody-antigen interaction. Alternatively, conjugated secondary antibodies can be used to bind to unconjugated primary antibodies (that detect the antigen) to visualize the interaction or amplify the signal.
The two primary antibody properties of interest are:
- Clonality – monoclonal vs. polyclonal
- Conjugation – fluorophore vs. enzyme
Monoclonal antibodies are produced by a single B cell population and recognize a single epitope. In contrast, polyclonal antibodies arise from multiple B cell populations and can recognize multiple epitopes on the same antigen. Monoclonal antibodies are therefore highly specific, whereas polyclonal antibodies are more resistant to changes in protein conformation because they can bind to multiple epitopes.
The type of conjugate bound to an antibody determines how the signal will be processed. Antibodies can be conjugated to fluorophores or to enzymes that process chromogenic substrates (ex. horseradish peroxidase processes 3,3' diaminobenzidine (DAB); ex. alkaline phosphatase processes 3-amino-9-ethylcarbazole (AEC)).
Below is a table summarizing briefly the respective advantages and disadvantages of various antibody clonalities and conjugations. For more information, refer to our previous article on monoclonal vs. polyclonal vs. recombinant antibodies.
| Antibody Type | Advantages | Disadvantages |
| Monoclonal Antibody | Highly specific to single epitope, therefore less likely to cross-react with other proteins and lower background signals. | Binds to single epitope therefore more susceptible to changes in antigen conformation (lower signal detection). |
| Polyclonal Antibody | Targets multiple epitopes of same antigen, therefore more resistant to changes in antigen conformation. | Multiple epitope targeting increases likelihood for non-specific signals (increased background). |
| Fluorophore-conjugated | The availability of multiple fluorophores with narrow emission ranges means multiple antigens can be visualized, with high spatial specificity. | Photobleaching of signals |
| Enzyme-conjugated | The chromogenic substrates provide strong signal that is longer lasting. | The signal is less spatially specific, and fewer signals can be visualized due to their broad emissions. |
In summary, the sample preparation method and antibody selection must be considered carefully to ensure quality results for an IHC experiment.
Editor's Note: Take advantage of the context filters on BenchSci to easily identify and review published IHC and/or IF data for your antibody selection.