Continuing from the previous article on the Principles of Blue Native-PAGE, I will elaborate on the technical details and nuances of running a BN-PAGE in this article.
I will also discuss the application of running a second dimension or 2D BN/SDS-PAGE to resolve the individual constituents of protein complexes.

Lysate Type and Mode of Lysis
The type of lysate and mode of lysis is crucial for maintaining complex stability for a Blue Native. Whether it is a whole cell lysate or subcellular fractionation of isolated organelles, the buffer conditions should be optimized for the protein complex of interest.
Typically, extracts of membrane bound organelles are prepared and normalized beforehand and are lysed the day of using a mild detergent and low salt concentration (<50mM NaCl) while avoiding potassium or divalent cations that may precipitate.
Our lab works with mitochondrial extracts lysed with digitonin at 3.0g/g (detergent/protein) in a 20mM Hepes pH 7.4, 10% glycerol and 50mM NaCl buffer. Wittig et al has a convenient table that shows the recommended detergent protein ratios for yeast, mammalian and bacteria cells. It is important that these be kept cold before and while the BN-PAGE is running to preserve the extract.
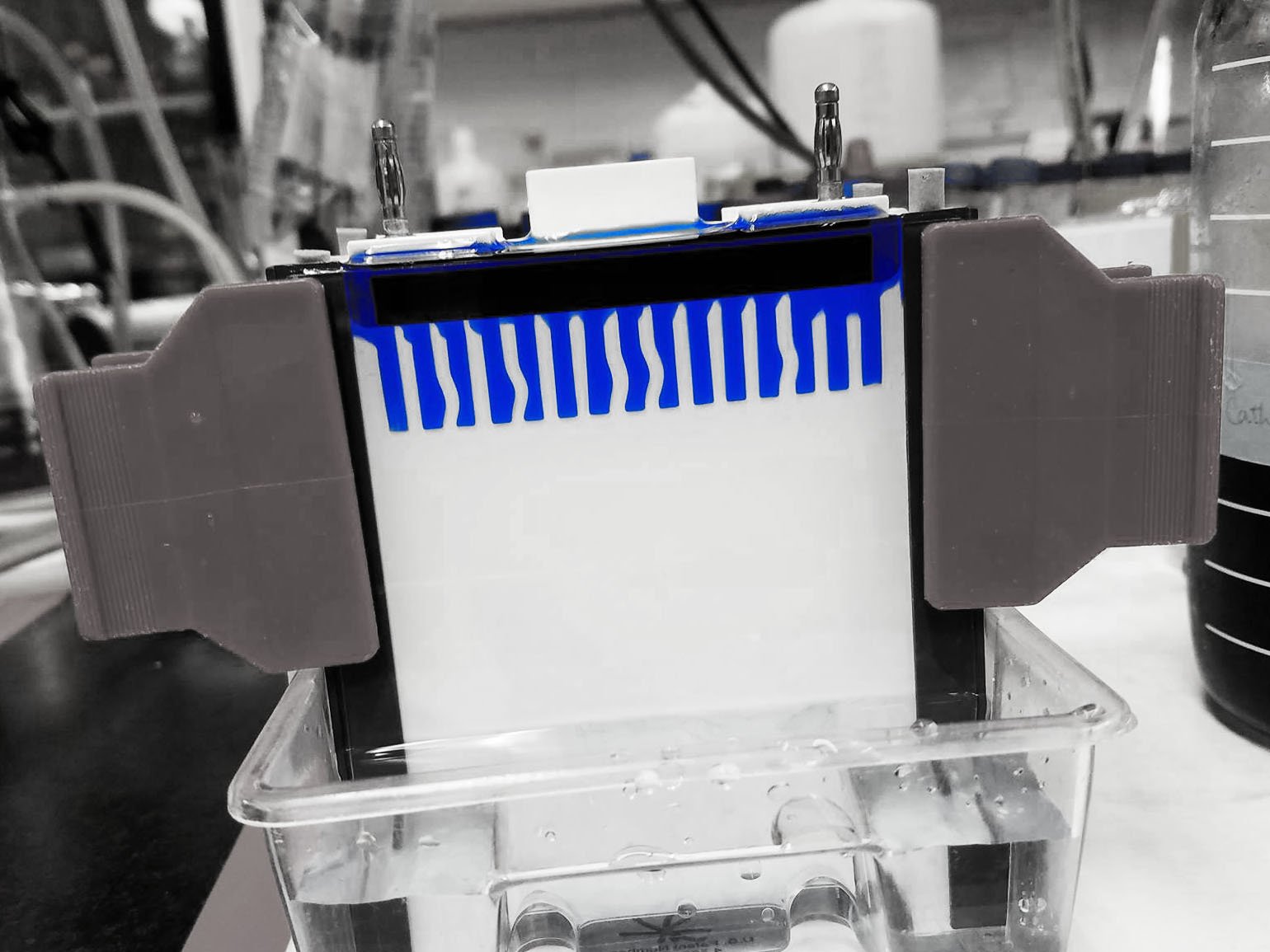
The Gradient Gel
The BN-PAGE gradient gel used for casting can be either imidazole or Bis-Tris pH7.0 with 6-aminocaproic acid. However, Wittig et al recommends an imidazole buffer because Bis-Tris can interfere with downstream protein assays.
All equipment involved in casting the gel should be thoroughly cleaned and rinsed so that no particulates or detergents disrupt BN-PAGE results. The gel can be poured at room temperature with a gradient gel casting system to ensure the lower and higher acrylamide percentage are thoroughly mixed. I have included a link to what a similar gradient gel casting system looks like in our lab.
The Cathode and Anode Buffers
Prepare your cathode and anode buffers beforehand and place them at 4°C until you are ready to load the gel. There should be two cathode buffers with and without 0.02% Coomassie blue G-250. Once your lysates are ready, a solution containing 5% Coomassie blue G-250 and 0.5M 6-aminohexanoic acid is added to the lysate to 8.0 (gram/gram) detergent/dye ratio. The high molecular weight ladder used for native protein gels should also be resuspended in the same solution.
Running the Gel
I typically load the gel in the 4 degree fridge before adding the cathode buffer with the Coomassie blue G-250. The blue from the dye can make it difficult to keep track of which wells have been loaded. Our lab runs the gels at a constant 100V with the blue cathode buffer for 1 hour or until the samples are well into the stacking gel.
After 1 hour the blue cathode buffer is poured out and replaced with the coloreless cathode buffer. This limits the amount of dye in the gel which can interfere with the transfer and immunoblotting. The electrophoresis is continued at a constant 12-15mA for an addition 1-2 hours. However, running conditions should be tailored for each gel system so that the BN-PAGE remains cold for the duration of the electrophoresis. Because the proteins are restricted by pore size, running at constant volts will cause the gel to run slower as the proteins progress through higher percentages of acrylamide.
Transferring
The BN-PAGE can be transferred on PVDF in the cold at 20V for at least 2 hours. The transfer buffer typically contains 20% methanol with a maximum concentration of 0.05% SDS to help with transfer of hydrophobic proteins. Once the transfer is completed, the high molecular weight ladder can be excised and transferred for Coomassie staining. The rest of the membrane can be washed with methanol to remove any excess Coomassie blue G-250 before immunoblotting.
Notes on Second Dimension (2D) BN SDS-PAGE
If you do not have access to clean antibodies or the antibody is unable to recognize the native form of your protein, a 2D BN/SDS-PAGE can be performed after the BN-PAGE. (Search through BenchSci for antibodies.)
A 2D BN/SDS-PAGE is also used to identify the components of specific protein complexes. This technique entails running a standard BN-PAGE, excising the desired lanes followed by embedding the lane horizontally in the stacking of an SDS-PAGE, sorting individual components of protein complexes by their molecular weight.
Though simple in concept, preparing the second dimension can be a bit tedious. To ease the transition between gels, the BN-PAGE lanes should be excised as clean and straight as possible. I usually use a fresh razor blade and cut in sections starting from the lowest part (highest percentage) of the gel. The high molecular weight ladder is also excised and fixed by destain or transferred to a membrane for reference later.
The excised lanes are then placed in a 1% SDS and 1% mercaptoethanol solution for 40 minutes at 60°C to denature the protein complexes. Once the denaturing is complete, the lane is thoroughly washed with water and placed horizontally on the top of a gel plate of the same size from the lowest acrylamide percentage (left) to highest (right). Be sure to leave room for two lanes to the left of the gel. These lanes will be used for the ladder and input controls.
Once the cassette is assembled with the gel lane in between the two plates, the separating gel can be poured into the opening on the left. Once the separating has polymerized, a stacking gel is poured in through the same opening, embedding your BN-PAGE lane into the stacking. Remember to place a comb that has been cut to only two lanes into the left opening of the gel for your ladder and input controls.
Make sure there are absolutely no bubbles in between your excised lane and the stacking that will disrupt downstream blotting. Making sure the lane is excised clean and straight without and jagged edges can help avoid this. Also, pouring the stacking very slowly and carefully while tilting the gel toward the right can help with the embedding. Be sure to load an appropriate amount of lysate for the input control so that the exposure should be similar to the amount of protein present in the complex.
Once the stacking has polymerized, the gel can be ran and transferred like a standard SDS-PAGE. Any antibodies used for SDS-PAGE can now be used to determine migration patterns for a BN-PAGE!
Check out BenchSci for published BN-PAGE data to help with your antibody selection!